New Developments in Battery Chargers
Abstract
Electronic equipment is increasingly becoming smaller, lighter, and more functional, thanks to the push of technological advancements and the pull from customer demand. The result of these demands has been rapid advances in battery technology and in the associated circuitry for battery charging and protection.
For many years, nickel-cadmium (NiCd) batteries have been the standard for small electronic systems. A few larger systems, such as laptop computers and high-power radios, operated on "gel-cell" lead-acid batteries. Eventually, the combined effects of environmental problems and increased demand on the batteries led to the development of new battery technologies: nickel-metal hydride (NiMH), rechargeable alkaline, lithium ion (Li+), and lithium polymer. These new battery technologies require more sophisticated charging and protection circuitry to maximize performance and ensure safety.
NiCd and NiMH Batteries
NiCd has long been the preferred technology for rechargeable batteries in portable electronic equipment, and in some ways, NiCd batteries still outperform the newer technologies. NiCd batteries have less capacity than Li+ or NiMH types, but their low impedance is attractive in applications that require high current for short periods. Power tools, for example, will continue to use NiCd battery packs indefinitely.
Though similar to NiCd types, NiMH batteries have greater capacity. This advantage is offset somewhat by the NiMH battery's higher self-discharge rate—approximately double that of the NiCd, which is relatively high to begin with (about 1% of capacity per day). Thus, NiMH batteries are not suitable for applications in which the battery is expected to hold its charge for a long time.
NiMH batteries also differ from NiCd batteries in the method required to fast charge them. Both types can be fast charged with a current equal to or greater than the capacity (C) in ampere hours. This technique allows you to charge a battery in about an hour or less. Because of internal losses, a battery charged at C for one hour cannot reach full capacity. For full capacity, you must either charge for an hour at more than C, or charge at C for more than an hour. Charging losses vary with the charging rate and from battery to battery.
When charging a NiCd battery, its terminal voltage peaks and then declines as the battery reaches capacity. An applied fast charge should therefore terminate when this voltage starts to drop (when ΔV/Δt becomes negative). Otherwise, the charging current delivers excess energy, which acts on the battery's electrolyte to dissociate water into hydrogen and oxygen gases. This results in a rise in internal pressure and temperature and a decrease in terminal voltage. If fast charging continues, the battery can vent (explode).
As a secondary or backup measure, NiCd and NiMH chargers often monitor the battery's temperature (in addition to its voltage) to ensure that fast charging is terminated before the battery is damaged. Fast charging should stop when a NiCd's ΔV/Δt becomes negative. For NiMH batteries, fast charging should stop when the terminal voltage peaks (when ΔV/Δt goes to zero).
Trickle charging is simple for NiCd and NiMH batteries. As an alternative to fast charging, the use of a small trickle current produces a relatively small rise in temperature that poses no threat of damage to the battery. There is no need to terminate the trickle charge or to monitor the battery voltage. The maximum trickle current allowed varies with battery type and ambient temperature, but C/15 is generally safe for typical conditions.
Lithium-Ion and Lithium-Polymer Batteries
A popular recent innovation in battery technology has been the introduction of lithium-ion (Li+) batteries. Li+ batteries have a higher capacity than previous rechargeable chemistries, such as NiCd and NiMH. The advantage of Li+ over NiMH is only 10% to 30% when measuring capacity as energy per unit volume, but volumetric capacity is not the only property to consider; weight is also important in a portable device. When measuring capacity as energy per unit mass, Li+ batteries are clearly superior (NiMH batteries are relatively heavy). Because they are lighter, Li+ batteries have nearly twice as much capacity per unit mass.
Li+ batteries also have many limitations. They are highly sensitive to overcharging and undercharging. You must charge to the maximum voltage to store maximum energy, but excessive voltage can cause permanent damage to a Li+ battery, as can excessive charge or discharge current. Discharging the battery also carries a caveat: repeated discharges to a sufficiently low voltage can cause a loss of capacity. Therefore, to protect the battery, the management circuit must limit the battery’s current and voltage when discharging as well as when charging. Most Li+ battery packs include some form of undervoltage- and overvoltage-disconnect circuitry. Other typical features include a fuse to prevent exposure to excessive current and a switch that open circuits the battery if high pressure causes it to vent.
Unlike NiCd and NiMH batteries, which require a current source for charging, Li+ batteries must be charged with a combination current-and-voltage source. To achieve the maximum charge without damage, most Li+ chargers maintain a 1% tolerance on the output voltage. (The slight additional capacity gained with a tighter tolerance is generally not worth the extra difficulty and expense required to achieve it.)
For protection, a Li+ battery pack usually includes MOSFETs that open circuit the battery in the presence of undervoltage or overvoltage. These protection MOSFETs also enable an alternative charging method (applying a constant current with no voltage limit) in which the MOSFETs are turned on and off as necessary to maintain appropriate battery voltage. The battery's capacitance helps to slow the rise of battery voltage, but use caution: battery capacitance varies widely over frequency, as well as from battery to battery.
In some applications, intermittent loads can exceed the main battery's power capability. A solution to this problem is to provide an additional, rechargeable battery to supply the excess current during a high-load transient. The main battery then recharges the auxiliary battery in preparation for the next transient. Small RF devices are a good example of this arrangement. This type of device generally runs from a single AA alkaline battery, but the load during transmission is too high for an AA battery to handle. An additional NiCd battery powers the transmitter, and it can be recharged when the transmitter is off, which is most of the time.
Replacing the NiCd AA battery with a Li+ version increases the total battery lifetime without sacrificing the needed current capacity. Lithium-polymer (LiPo) batteries evolved technologically from lithium-ion batteries. Compared to their Li+ counterparts, LiPo cells have an even higher energy capacity per unit mass, less series resistance, and have the added advantage of mechanical flexibility. This flexibility allows the battery designer to shape it in any shape that is convenient for the application.
LiPo batteries share many characteristics with Li+, and thus the same care must be taken when monitoring them to maximize performance and ensure safety.
Choosing the Right Battery Chemistry
Each battery chemistry has advantages and disadvantages; choosing the right chemistry for each application requires careful planning. Table 1 below summarizes battery parameters for several chemistry types. This information should be considered a summary only, and using actual data from battery manufacturers is recommended.
| Parameter | Lead Acid | NiCd | NiMH | Alkaline | Li-Ion | Li-Polymer |
| Cell Voltage (V) | 2.0 | 1.2 | 1.2 | 1.5 | 3.6 | 3.7 |
| Relative Cost | Low | Moderate | High | Very low | Very high | Very high |
| Internal Resistance | Low | Very low | Moderate | Varies | High | Low |
| Self Discharge (%/month) | 2% to 4% | 15% to 30% | 18% to 20% | 0.3% | 6% to 10% | 5% |
| Cycle Life (Charge Cycles to Reach 80% of Rated Capacity) | 500 to 2000 | 500 to 1000 | 500 to 800 | Low | 1000 to 1200 | > 1000 |
| Overcharge Tolerance | High | Medium | Low | Medium | Very low | Very low |
| Energy Density by Volume (Wh/L) | 70 to 110 | 100 to 120 | 135 to 180 | 220 | 280 to 320 | ~400 |
| Energy Density by Weight (Wh/kg) | 30 to 45 | 45 to 50 | 55 to 65 | 80 | 90 to 110 | 130 to 200 |
Cradle Chargers
For cell phones and many other devices, the preferred battery-charging method involves the use of a separate "cradle charger" into which you place the device or the battery pack (like a baby in its cradle). Because the charger unit is separate, its generated heat is less of a concern than it would be if the charger were integrated into the device.
The simplest circuit for use in a cradle charger is usually a linear-regulator charger. Linear regulators drop the difference voltage (between the DC power source and the battery) across a pass transistor operating in its linear region (hence the name linear regulator). However, the dissipated power (the charging current times the drop across this transistor) can cause overheating if the charger is confined to a small space without airflow.
For example, consider a four-cell NiCd battery charged at 1A. NiCd batteries usually terminate charging at approximately 1.6V or 1.7V per cell, but the voltage can be as high as 2V per cell, depending on the battery's condition and its charging rate. The DC source voltage must therefore be greater than 4 × 2V = 8V. The voltage level of cells in a fully discharged battery can measure as low as 0.9V each; in this case, the battery voltage is 4 × 0.9V = 3.6V. If the DC source is 8V, the pass transistor sees 8V - 3.6V = 4.4V.
When charging a fully discharged battery, the dissipated power is 4.4W in the charger and 3.6W in the battery—an efficiency of only 45%! The actual efficiency is even lower, because the DC source voltage must be higher than 8V to account for dropout voltage in the pass transistor and tolerance in the source.
A linear, single-cell Li+ charger is suitable for use in a cradle charger (Figure 1). It drives an external power transistor (Q1) that drops the source voltage down to the battery voltage. The external transistor accounts for most of the circuit's power dissipation; therefore, the controller temperature remains relatively constant. The result is a more stable internal reference, yielding a more stable battery-voltage limit.
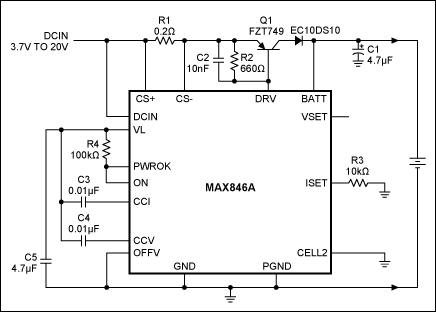
Figure 1. Designed for single lithium-ion cells, this battery-charging circuit is ideal for use in a stand-alone cradle charger.
R1 and R3 determine the output current. R1 senses the charging current, and R3 sets the level at which the current is regulated. Current out of the ISET terminal is equal to 1/1000 of the voltage between CS+ and CS-. The current regulator controls the ISET voltage at 2V; in this case, the current limit [2000/(R3 * R1)] is 1A.
Control loops for the voltage and current limits have separate compensation points (CCV and CCI), which simplifies the task of stabilizing these limits. The ISET and VSET terminals allow for adjustment of the current and voltage limits.
Built-In Battery Chargers
For some larger systems, including laptop computers, the battery charger is built in as part of the system. The charger's efficiency in this arrangement is critical—not to ensure maximum energy transfer, but simply to minimize heat generation. Heat elevates temperature, and operation at elevated temperatures shortens a battery's life. Because this application requires high efficiency over the entire battery-voltage range, the charger should rely on a switching regulator, whose power dissipation is relatively low and independent of the input-to-output voltage drop.
The main drawback of switching regulators is the need for a passive inductor/capacitor filter, which converts the switched output voltage to a DC level suitable for the battery. In some cases, the battery capacitance is sufficient to replace the capacitor in this filter; however, as mentioned earlier, a battery's capacitance can vary greatly with frequency. Characterize it carefully before committing to a design.
Another drawback of switching regulators is the noise generated by their switching action. This problem can usually be avoided with proper layout techniques and shielding. For applications in which certain frequencies should be avoided, many switching chargers can be synchronized to an external signal—a capability that allows you to shift the charger's switching noise away from sensitive frequency bands.
A linear regulator is generally larger than an equivalent switching regulator because it dissipates more power and requires a larger heatsink. Consequently, the extra time necessary to design a smaller, more efficient switching charger is usually justified. One such design is the 4-cell NiCd/NiMH charger shown in Figure 2. It has no provision for terminating the charge; it operates in conjunction with a controller that monitors voltage across the battery and shuts off the charger when conditions are met. Many systems already include a controller suitable for this purpose. If your system does not have one, you will need a low-cost, stand-alone microcontroller (µC) that includes an on-board analog-to-digital converter (ADC). A number of such µCs are available.
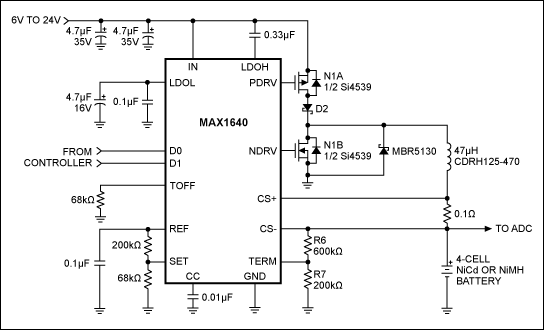
Figure 2. This 4-cell NiCd or NiMH battery charger can be incorporated into a larger system.
The charger IC (MAX1640) chops the input voltage using a switching transistor (N1A) and a synchronous rectifier (N1B). This chopped voltage is placed across the inductor to form a current source. When the charger is turned off, diode D2 prevents current flow from the charged battery back into the voltage source.
In addition to "off," the MAX1640 operates in one of three modes as determined by the digital inputs D0 and D1: fast charge, pulse-trickle charge, and top-off charge (Table 2). In fast-charge mode, the charging current is 150mV divided by the current-sense resistor value (0.1Ω), or 1.5A in this case. In top-off-charge mode, the voltage at SET produces 25.4% of the fast-charge current, or 381mA in this case. The current in pulse-trickle-charge mode is the same as in top-off mode, but it is pulsed with a 12.5% duty cycle. Frequency is determined by the resistor connected at TOFF (68kΩ). In this case, the frequency is 3.125MHz/R3 = 46Hz. The average pulse-trickle current is therefore 0.125 × 381mA = 47.6mA.
| D0 | D1 | Mode | Output Current |
| 0 | 0 | Off | — |
| 0 | 1 | Top-off charge | VSET/(13.3RSENSE) |
| 1 | 0 | Pulse-trickle charge | VSET/(13.3RSENSE) (12.5% duty cycle) |
| 1 | 1 | Fast charge | VREF/(13.3RSENSE) |
The circuit in Figure 2 should terminate a charge when ΔV/Δt equals zero or becomes negative (according to whether a NiMH or NiCd battery is being charged). However, if termination fails to occur, the circuit imposes a secondary voltage limit to prevent the battery voltage from rising too high. As an absolute maximum, the charging voltage for NiCd and NiMH batteries should not exceed 2V per cell, or 8V for the 4-cell battery in this circuit. R6 and R7 establish this voltage limit as VLIMIT = VREF [R7/(R6 + R7)].
A similar circuit charges two Li+ cells in series (Figure 3). It differs mainly in the accuracy of its charging voltage, which is better than the 1% required by Li+ batteries. Also unlike the Figure 2 charger, this one employs an n-channel MOSFET for the high-side switching transistor. When turned on, this transistor's source and drain voltages are approximately equal to VIN, but the gate voltage must be higher than VIN to allow the use of inexpensive n-channel MOSFETs. This elevated gate drive is achieved by charging C7 and adding its voltage to VIN.
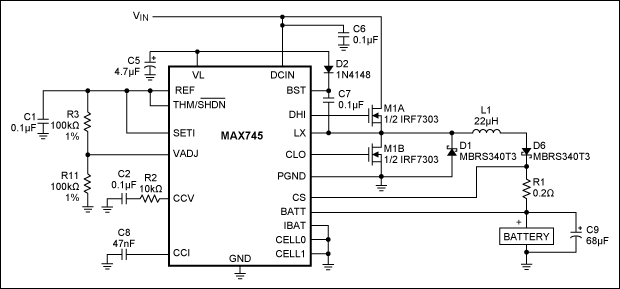
Figure 3. This charger generates a 1%-accurate charging voltage suitable for charging two lithium-ion batteries in series.
Charging current for the circuit shown in Figure 3 is determined by current-sense resistor R1: 185mV/R1 = 925mA for the 200mΩ value shown. This current can be adjusted linearly to lower values by varying the voltage at the SETI terminal. Similarly, you can adjust VOUT by varying the voltage at the VADJ terminal. Because varying VADJ over its full range (0V to 4.2V) changes VOUT by only 10% (0.4V per cell), you can achieve better than 1% output accuracy using 1% resistors. (One-percent-accurate resistors degrade the output accuracy by only 0.1%.).
Terminals CELL0 and CELL1 set the battery's cell count as shown in Table 3. (VL indicates the 5V level that powers the chip.) The charger can handle as many as four Li+ cells in series. Though not shown in Figure 3, the MAX745 can also terminate charging upon reaching a temperature limit monitored by a thermistor. When the battery temperature exceeds this limit (determined by an external resistor and thermistor connected to the THM/active-low SHDN terminal), the charger shuts off. Hysteresis associated with this threshold enables the system to resume charging when a declining battery temperature causes the THM/active-low SHDN voltage to fall 200mV below its 2.3V threshold.
| CELL0 | CELL1 | Number of Cells |
| GND | GND | 1 |
| VL | GND | 2 |
| GND | VL | 3 |
| VL | VL | 4 |
Smart-Battery Chargers
Smart batteries represent a new technology that is helping designers and consumers alike. Smart-battery packs include a controller that can "talk" through its serial port to tell an external charger what kind of charging routine the battery requires. This arrangement helps designers, because they can design a single charger that handles all batteries compliant with the smart-battery standard.
Smart batteries also benefit consumers, who can replace a given battery without regard to its type, as long as the replacement is smart-battery compliant. The smart-battery specification allows any manufacturer to participate in the market, and the resulting competition leads to standard products and lower prices.
The smart-battery specification was defined by a consortium of companies that manufacture batteries, computers, and related products. It defines the way the battery pack connects to the host system and the way it communicates with the host. It communicates via a two-wire serial interface known as the System Management Bus (SMBus), which is derived from the I2C protocol. A large base of I2C-compliant µCs capable of controlling peripherals on the SMBus is already available.
Smart batteries also provide an elegant solution to the problem of fuel gauging. In a system run by ordinary noncommunicating batteries, the host knows the state of the battery only when it has been fully charged or discharged. Smart batteries, on the other hand, remember their charge state. When such batteries are switched in and out of the host, the fuel gauge is able to maintain the same level of accuracy as it would under continuous operation.
In the smart-battery-compliant charger shown in Figure 4, the controller IC includes an SMBus interface that allows it to communicate with the host computer and the smart battery under charge. Because the switching regulator and its small, power-efficient current-sense resistor cannot achieve a 1mA (min) resolution in charging current, the first 31mA (5 LSBs) of output current are supplied by an internal linear current source.
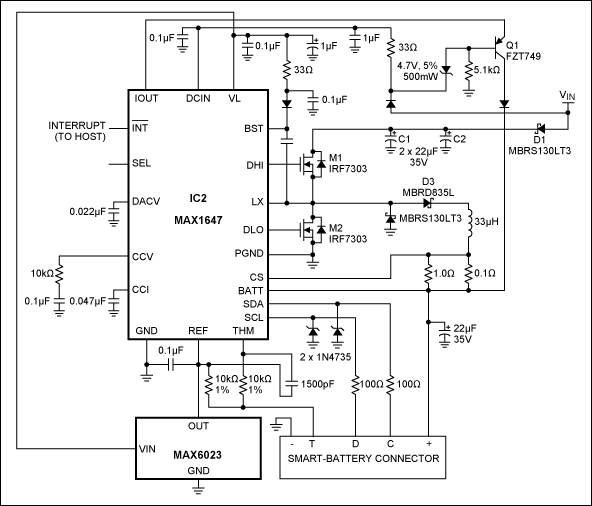
Figure 4. This charger is compliant with the smart-battery specification, and communicates with the host computer and a smart battery via the SMBus interface.
To preserve high efficiency (89%), the system activates a switch-mode current source when programmed for output currents of 32mA or more. however, the linear source remains on to ensure monotonicity in the output current regardless of the current-sense resistor's value or offset in the current-sense amplifier. Transistor Q1 off-loads an otherwise heavy power dissipation in the internal linear regulator, which occurs when the input voltage is much greater than the battery voltage. Q1's base is held approximately 5V below the input voltage. Voltage across the internal current source is less than 5V; therefore, power dissipation in the current source remains below 160mW.
A diode (D3) is placed in series with the inductor to prevent a flow of reverse current out of the battery. The MAX1647's high switching frequency (250kHz) permits the use of a small inductor. The circuit accepts inputs as high as 28V, and provides pin-selectable maximum output currents of 1A, 2A, and 4A.