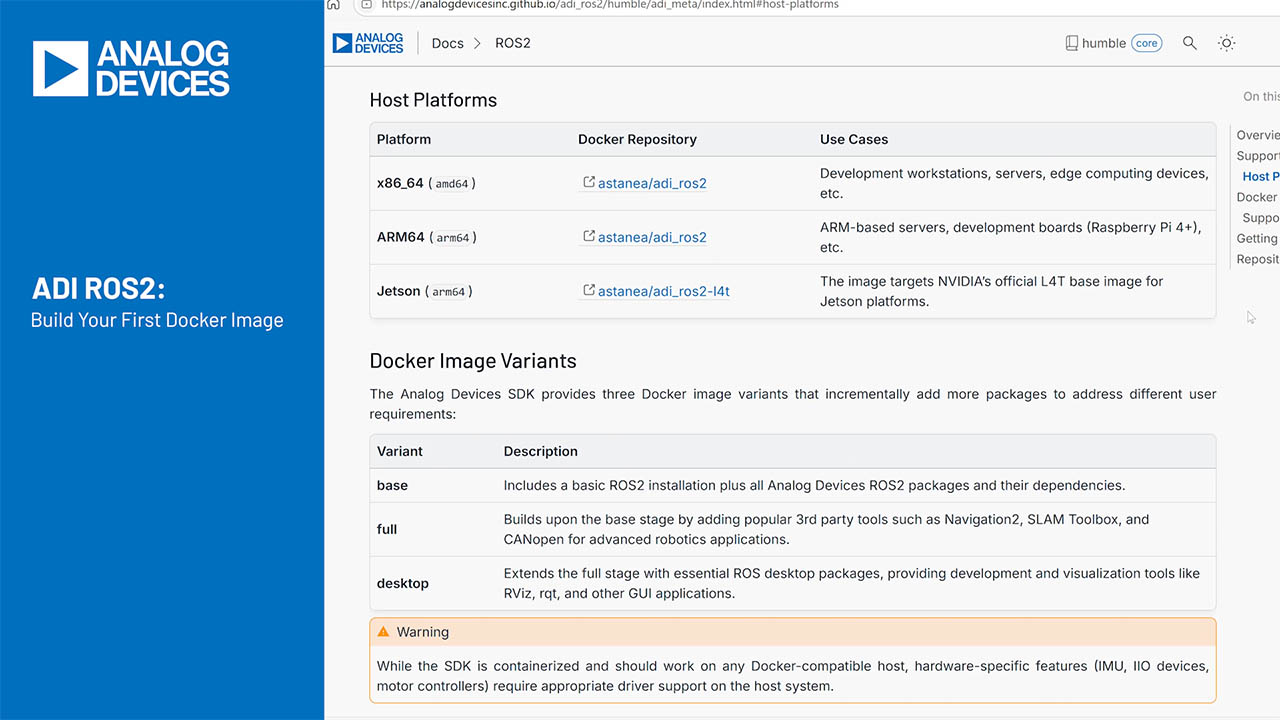
Guidelines for the Opto-Mechanical Integration of Heart-Rate Monitors in Wearable Wrist Devices
Abstract
The opto-mechanical integration of light-emitting and light-sensing elements into bio-sensing wrist wearables is a fundamental step in the wearable design process. The quality of the signal can be greatly affected by choosing components and geometries that minimize crosstalk and maximize signal to noise. This application note discusses the optical and mechanical aspects to be considered for optimal performance. The optical portion focuses on the interaction of light with the skin and blood as well as selection of LEDs and photodetectors. The mechanical portion provides suggestions that increase coupling between the optics and the skin.
Introduction
Wrist-based wearables are gaining prominence with customers who want to track their physiological parameters during fitness, daily activity, and sleep. These physiological parameters can be obtained noninvasively by using optical sensors to detect the heart-rate signal. This technology has been established in the medical sector and can now be transferred to wearable wrist applications.
Theory of Operation
Photoplethysmography (PPG) is the principle on which optical sensors measure heart rate. As the heart pumps, the volume of blood transported in the arteries changes. More blood flows through the arteries when the heart expels blood (systolic phase), and less blood flows when the heart draws blood (diastolic phase). When the blood volume changes between systolic and diastolic heart beats, it results in a change in the optical absorption coefficient of the arterial layer. By optically illuminating the tissue and measuring the transmitted light, the absorption change due to the blood volume change can be determined and the heart-rate pulsatile signal can be recovered.
On certain regions of the body such as the wrist, transmissive heart-rate measurements are logistically difficult, so reflective measurements are used. Reflective heart-rate monitors consist of a light source and a detector arranged in the same plane (Figure 1). The light emitted penetrates the skin, tissue, and blood vessels and is either absorbed, scattered, or reflected. A small portion of the emitted light eventually reaches the photodetector. As the volume of blood in the arteries changes with every heartbeat, the fraction of light absorbed and, subsequently, the strength of the detector signal change.

Figure 1. The principle of reflective optical-pulse measurements.
Interaction of Light with the Skin
The skin consists of three main layers from the surface: the blood-free epidermis layer (100µm thick), vascularized dermis layer (1mm to 2mm thick), and subcutaneous adipose tissue (1mm to 10mm thick depending on the body site). Typically, the optical properties of these layers are characterized by absorption (µa), scattering µs coefficients, and the anisotropy factor (g).
The absorption coefficient characterizes the average number of absorption events per unit path length of photons traveling in the tissue. The main absorbers in the visible spectral range are melanin, which is blood composed of oxyhemoglobin (Hb), deoxyhemoglobin (HbO2), and lipids. In the IR spectral range, the absorption of water dominates the absorption properties of the skin dermis.
Figure 2 is a planar seven-layer optical model of human skin. The layers included in this model are the following:
- Stratum corneum
- Two layers of living epidermis.
- The first layer contains the papillary dermis and superior blood net dermis
- The second layer contains the reticular dermis and inferior blood net dermis
- Subcutaneous adipose tissue

Figure 2. Seven-layer skin model. The first and outermost layer is the stratum corneum, and the innermost layer is the subcutaneous adipose tissue or fat layer.
Table 1 presents the thickness of the layers as well as typical ranges of blood, water, and melanin contents, and refractive indices of the layers.
| Layer | Thickness | Volume Fraction | Refractive Index | |||
| t(µm) | θ(blood) | θ(water) | θmel | n | ||
| 1 | Stratum corneum | 20 | 0 | 0.05 | 0 | 1.40 |
| 2 | Epidermis | 80 | 0 | 0.2 | 0.01 – 0.10 | 1.40 |
| 3 | Papillary dermis | 150 | 0.0024 | 0.5 | 0 | 1.39 |
| 4 | Superior blood net dermis | 150 | 0.0060 | 0.6 | 0 | 1.39 |
| 5 | Reticular dermis | 1000 | 0.0024 | 0.7 | 0 | 1.41 |
| 6 | Inferior blood net dermis | 600 | 0.0120 | 0.7 | 0 | 1.41 |
| 7 | Subcutaneous fat | 8000 | 0.0012 | 0.7 | 0 | 1.44 |
The heart-rate pulsatile signal originates from the arterial bed that is lying down in the inferior blood net dermis layer, which is the sixth layer in the seven-layer tissue model in Table 1. The absorption spectrum of this layer can be calculated using the absorption spectra of tissue constituents and its corresponding volume fractions by using the following equation:
µa (λ) = (Sµa,Hb(λ) + (1−S)µa,HbO2(λ))θblood + µa,mel(λ)θmel + µa,water(λ)θwater + µa,lip(λ)θlip
where µa,mel, θmel, µa,water, θwater, µa,lip, θlip are the absorption coefficients and volume fractions of melanin, water, and lipids, respectively. µa,Hb and µa,HbO2 are the absorption coefficients of oxyhemoglobin and deoxyhemoglobin, and θblood is the volume fraction of blood. S is the blood oxygen saturation coefficient, typically around 95% in healthy individuals.
Use Equation 1 and the measured absorption spectra for the tissue constituents[1] to calculate the absorption coefficient in the inferior blood net dermis as a function of wavelength. Figure 3 plots the results.
In Figure 3, the peak absorption coefficients correspond to wavelengths around 540nm and 570nm. At these wavelengths, the absorption change due to the blood volume change is greatest, and the photodiode measures the strongest pulsate signal.

Figure 3. Absorption coefficient in the inferior blood net dermis layer as a function of wavelength.
Component Selection
LED Wavelengths and Efficiency
For acquiring the best PPG signal (i.e., largest AC heart-rate signal), the LED illumination wavelength should be as close as possible to the absorption peaks of the blood HbO2 at around 540nm and 570nm (Figure 3). However, due to the known "green gap" range in the LED’s luminous efficacy around 560nm, commercially available LEDs are very dim at these two desired wavelengths and, hence, not very useful for practical applications where high signal-to-noise ratio (SNR) is required. Therefore, green LEDs emitting around 530nm are utilized in most commercial PPG sensors available on the market.
Maxim Integrated has explored illumination wavelengths at both sides of the green absorption peak, the widely used true-green LEDs at 530nm and yellow LEDs at 590nm. While both wavelengths are available with large luminous efficacy from multiple LED vendors, we have found the OSRAM® PointLED[2] product line to offer the most suitable form-factor for building Maxim® wrist PPG sensor wearable prototypes.
Photodiode
The photodiode is one of the most critical component selection choices in a wearable heart-rate monitor because it is the first stage in the receiving path of the system. There are many photodiode options available in the broad market, so it is important to choose one with high responsivity at key operating wavelengths or ranges.
Responsivity is a measure of the electrical output per optical input and is often expressed in current produced per watt of incident radiant power (A/W). A high-responsivity device detects the small heart-rate signals returning from scattering inside the wrist tissue. Si PIN photodiodes have the largest responsivity in the visible/NIR wavelength range and are available from many manufacturers. Vishay® and OSRAM Si PIN photodiodes are particularly useful for biosensing wrist wearables due to their small form factor[3,4].
Optomechanical Design Considerations
Overview
Designing a good optical PPG solution is very complex and is often underestimated. Successful integration of the optical components maximizes both the signal received by the sensor and the signal-to-noise parameter. Increase the latter by maximizing the signal that penetrates deep enough into the skin to detect a PPG signal while minimizing crosstalk from signals on the sensor from sources other than the PPG signal.
Figure 4 shows a typical opto-mechanical integration design.

Figure 4. Typical opto-mechanical design.
The LEDs and photodiode are encapsulated in a transparent material to provide a moisture barrier and to interface between the optical components and the wrist. Barriers between the LEDs and the photodiode provide optical isolation to ensure that only light that has traversed through the skin tissue is detected by the photodiode. The entire assembly protrudes from the bottom of the wrist band to ensure tight contact with the skin.
Waterproofing/Sealing Components
As with any wrist wearable design, customers need some sort of sealant for the optical design. A sealant provides waterproofing properties and increases the signal received by the sensor. Choose a sealant that has an index of refraction close to that of human skin (approximately 1.4) to minimize Fresnel reflections that cause transmission losses. Additionally, a sealant that provides some “give” can increase the contact area and pressure with the skin. Silicones are commonly used sealants. Table 2 lists good silicone candidates based on the encapsulation material and their characteristics.
| Encapsulation Comparison Table | |||||||
| Manufacturer | Product Name | Color | Viscosity (cP) | Index of Refraction | Transmission (3.2mm Thick) | Hardness | Comments |
| Dow Corning | Sylgard 184 Silicone Elastomer | Colorless | 3,500 | 1.4118 at 589nm 1.4225 at 632.8nm |
97% at 532nm 96% at 880nm |
Durometer Shore 43 | High transmission, flexible elastomer |
| Dow Corning | EI-1184 Optical Encapsulant | Clear | 5,300 | 1.42 at 632.8nm | 93% at 380nm 94% at 450nm 94% at 760nm |
Durometer Shore A 61 | Cures to flexible elastomer, cure time can be decreased with heat |
| Dow Corning | MS-1002 Moldable Silicone | Optically clear | 26,250 | 1.41 at 632.8nm | 89% at 380nm 91% at 450nm 94% at 760nm |
Durometer Shore A 72 | Moldability allows for more complex designs |
| Dow Corning | MS-1003 Moldable Silicone | Optically clear | 42,300 | 1.41 at 632.8nm | 91% at 380nm 92% at 450nm 93% at 760nm |
Durometer Shore A 51 | Moldability allows for more complex designs |
| Dow Corning | MS-4002 Moldable Silicone | Optically clear | 25,000 | 1.42 at 632.8nm | 89% at 380nm 92% at 450nm 93% at 760nm |
Durometer Shore A 84 | Moldability allows for more complex designs |
| Dow Corning | IMS-4007 Moldable Silicone | Optically clear | 10,500 | 1.41 at 632.8nm | 91% at 380nm 93% at 450nm 94% at 760nm |
Durometer Shore A 70 | Moldability allows for more complex designs |
Alternately, a transparent cover can provide a moisture barrier and interface between the optical components and the skin. The chosen material for the transparent cover should have high transmission (>90%) in the wavelengths utilized to maximize the light emitted into the skin and the signal returning from the skin. To minimize transmission loss, the transparent cover should be as thin as possible while still providing the robustness to withstand normal wear and tear. Additionally, the transparent cover should have an index of refraction close to that of human skin (~1.4) to minimize transmission losses due to Fresnel reflections.
Due to mechanical tolerances, an air gap is needed between the optical components and the bottom of the transparent cover. However, introduction of an air gap allows light to reflect off the bottom of the cover glass and hit the photodetector. This unwanted light has not traversed skin and degrades the heart-rate monitor’s performance. As the air gap is increased, the crosstalk increases. Thus, the air gap should be kept to a minimum. Additionally, an increase in air gap increases the path length necessary for the signal to reach the sensor and thus decreases the total signal received by the sensor. This is yet another reason the air gap between the sensor/LEDs should be minimized. We recommend not exceeding an air gap of 0.8mm to ensure acceptable performance.
We have found Corning® Gorilla® Glass fits both these criteria. The index of refraction of Corning Gorilla Glass is 1.5, the transmission between typical operating wavelengths (532nm and 880nm) is over 91%, and the glass provides a structurally sound cover with a thickness as thin as 200µm. Other potential material candidates include acrylics and polycarbonates.
Crosstalk-Suppressing Features: Light Barriers
Crosstalk is a signal incident on the photodiode that has not traversed through any skin layers. High levels of crosstalk drown out the pulsating heart-rate signal, rendering the wearable monitor incapable of effectively measuring PPG. Thus, crosstalk between the LED emission and the photodetector should be minimized for optimal performance. Use physical absorbing light barriers to maintain a low level of crosstalk. Figure 4 shows an example barrier.
Mesa: Increasing Contact with Skin
A raised mesa is a commonly used technique to help mitigate motion artifacts by ensuring proper coupling between the device and the skin. Figure 4 shows the mesa concept ensures the proper skin contact required for heart-rate detection.
Spacing Between LEDs and Sensor
One of the major design considerations in building a reflectance heart-rate monitor is determining the optimum separation distance between the LEDs and the photodiode. This distance should be selected such that PPG signals with both maximum and minimum pulsatile components can be detected. These pulsatile components depend not only on the amount of arterial blood in the illuminated tissue but also on the systolic blood pulse strength in the peripheral vascular bed.
There are two techniques that can enhance the quality of the PPG signal. One technique is to use a large LED driving current, which increases the effective penetration depth of the incident light through the higher light intensity. For a given LED-photodiode separation, using higher levels of incident light results in illuminating a larger pulsatile vascular bed. As a result, the reflected plethysmogram contains a larger pulsate signal component. However, in practice, the LED driving current is limited by the manufacturer to a specified maximum power dissipation.
The alternate method is to place the photodiode close to the LEDs. However, if the photodiode is too close to the LEDs, the photodiode becomes saturated by the large non-pulsatile components obtained by the multiple scattering of incident photons by the blood-free stratum corneum and epidermal layers in the skin.
For a constant LED intensity, the total light detected by the photodiode decreases roughly exponentially as the radial distance between the LEDs and the photodiode is increased. In other words, the effect of LED-photodiode separation on the reflected pulse amplitude of both green and yellow plethysmograms is decreased with the increase in separation. Thus, selecting the separation distance has its tradeoffs. A plethysmogram with a larger pulsate signal component can be achieved by placing the photodetector (PD) farther apart from the LEDs, but a higher LED driving current is needed to overcome absorption due to increased optical path length.
Simulated Comparison of LED-Photodiode Separation
To evaluate the effects of the LED-photodiode separation, there are two key figures of merit for PPG measurements: collection efficiency (CE) and perfusion index (PI).
CE is the fraction of power back on the photodiode for a given LED output. This optical signal incident on the photodiode is converted into current and consists of a large constant DC and a small variable AC component. The DC component contains no heart-rate information, while the AC corresponds to the pulsing arterial blood [5] (Figure 5).
The PI (defined as the ratio of AC to DC) is the ratio of the pulsatile blood flow to the non-pulsatile static blood flow in the peripheral tissue. The PI indicates the pulse strength at the sensor site. The higher the PI is, the better the performance is. The perfusion index depends on the path length through the inferior blood net dermis (l) and the change in the absorption coefficient (Δµ), which are wavelength dependent, as shown in the following expression:
PI= AC/DC = lΔµ
The perfusion index varies depending on skin type, motion artifacts, ambient light, fitness levels, and fat content in the body. In wrist-based applications, PI values range from 0.02% for a very weak pulse to 2% for an extremely strong pulse.
Because a good PPG signal is a tradeoff between total power and PI, the figure of merit to examine when determining the optimal LED-photodiode spacing is the product of the CE and PI (CE × PI). This quantity is proportional to the AC signal strength, and a higher CE × PI value corresponds to a greater AC signal.

Figure 5. Light absorption of the skin and corresponding DC and AC levels.
Ray trace simulation demonstrates the effects of LED-photodiode separation on the PI. The simulation uses a Monte Carlo method to trace optical rays propagating in complex, inhomogeneous, randomly scattering, and absorbing media. The simulated geometry consists of 1mm x 1mm active area detectors distanced 1mm to 10mm from an Lambertian emitting LED. The seven-layer model of the skin is placed above the LED and PDs. Figure 6 shows the simulation setup.

Figure 6. Simulation setup to determine optimal LED-photodiode separation.
The simulation determines the CE for a given wavelength and LED-photodiode spacing. Post-processing the simulation results yields the corresponding path length in the inferior blood net dermis layer. Knowing the path length (l) and the change in the absorption coefficient (Δµ), the PI can be calculated by using Equation 2.
The simulation results are given in Figure 7, Figure 8, and Figure 9 for 530nm, 560nm, 574nm, and 590nm. From Figure 9, it is evident that up to 3mm LED-photodiode separation, 574nm yields the highest PPG signal. Above 3mm separation, the 590nm PPG signal quality outperforms other wavelengths.

Figure 7. Collection efficiency as a function of LED-photodiode spacing.

Figure 8. Perfusion index as a function of LED-photodiode spacing.

Figure 9. Product of the collection efficiency and the perfusion index as a function of LED-photodiode spacing.
Maxim offers ICs that are suited for wearable, wrist-based heart-rate detection applications. The MAX86140/MAX86141 devices are complete integrated optical data acquisition systems, ideal for optical pulse oximetry and heart-rate detection applications. Both include high-resolution optical readout, signal-processing channels with ambient-light cancellation, and high-current LED driver DACs to form a complete optical readout signal chain. The MAX86140 consists of a single optical readout channel, while the MAX86141 has two optical readout channels that can operate simultaneously. Both the MAX86140 and MAX86141 have three LED drivers and are well suited for a wide variety of optical-sensing applications.
While the MAX86140/MAX86141 devices take care of the data acquisition, the customer must decide how to integrate the LEDs and photodetector into their industrial design.
Summary
Small, powerful analog front-end electronics are facilitating the ability to incorporate biosensing functionalities such as heart-rate monitoring in consumer wrist wearables. The performance of these wearable sensors depends greatly on careful optical component selection and opto-mechanical integration into the end customer’s design. Key parameters to consider when choosing the optical components are the wavelength and luminous efficacy for the LED, wavelength, and responsivity of the photodiode. To ensure the highest quality signal, encapsulation, crosstalk-suppressing barriers, and careful choice of LED-photodiode separation should be considered.