Battery Conditioner Extends the Life of Li-Ion Batteries
Introduction
Li-Ion batteries naturally age, with an expected lifetime of about three years. But, that life can be cut very short—to under a year—if the batteries are mishandled. It turns out that the batteries are typically abused in applications where intelligent conditioning would otherwise significantly extend the battery lifetime. The LTC4099 battery charger and power manager contains an I2C controlled battery conditioner that maximizes battery operating life, while also optimizing battery run time and charging speed (see Figure 1).
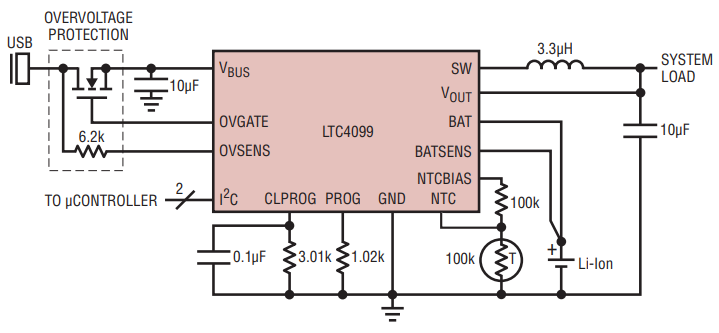
Figure 1. The LTC4099 with I2C Controlled Battery Conditioner
The Underlying Aging Process in Li-Ion Batteries
Modern Li-Ion batteries are constructed of a graphite negative terminal, cobalt, manganese or iron phosphate positive terminal and an electrolyte that transports the lithium ions.
The electrolyte may be a gel, a polymer (Li-Ion/Polymer batteries) or a hybrid of a gel and a polymer. In practice, no suitable polymer has been found that transports lithium ions effectively at room temperature. Most ‘pouch’ Li-Ion/ Polymer batteries are in fact hybrid batteries containing a combination of polymer and gel electrolytes.
The charge process involves lithium ions moving out of the negative terminal material, through the electrolyte and into the positive terminal material. Discharging is the reverse process. Both terminals either release or absorb lithium ions, depending on whether the battery is being charged or discharged.
The lithium ions do not bond with the terminals, but rather enter the terminals much like water enters a sponge; this process is call ‘intercalation.’ So, as is often the case with charge-based devices such as electrolytic capacitors, the resulting charge storage is a function of both the materials used and the physical structure of the material. In the case of the electrolytic capacitor, the foil is etched to increase its surface area. In the case of the Li-Ion battery the terminals must have a sponge-like physical makeup to accept the lithium ions.
The choice of positive terminal material (cobalt, manganese or iron phosphate) determines the capacity, safety and aging properties of the battery. In particular, cobalt provides superior capacity and aging characteristics, but it is relatively unsafe compared to the other materials. Metallic lithium is flammable and the cobalt positive terminal tends to form metallic lithium during the discharge process. If several safety measures fail or are defeated, the resulting metallic lithium can fuel a “vent with flame” event.
Consequently, most modern Li-Ion batteries use a manganese or iron phosphate-based positive terminal. The price for increased safety is slightly reduced capacity and increased aging.
Aging is caused by corrosion, usually oxidation, of the positive terminal by the electrolyte. This reduces both the effectiveness of the electrolyte in lithium-ion transport and the sponge-like lithium-ion absorption capability of the positive terminal. Battery aging results an increase of the battery series resistance (BSR) and reduced capacity, as the positive terminal is progressively less able to absorb lithium ions.
The aging process begins from the moment the battery is manufactured and cannot be stopped. However, battery handling plays an important role in how quickly aging progresses.
Conditions that Affect the Aging Process
The corrosion of the positive terminal is a chemical process and this chemical process has an activation energy probability distribution function (PDF). The activation energy can come from heat or the terminal voltage. The more activation energy available from these two sources the greater the chemical reaction rate and the faster the aging.
Li-Ion batteries that are used in the automotive environment must last 10 to 15 years. So, suppliers of automotive Li-Ion batteries do not recommend charging the batteries above 3.8V. This does not allow the use of the full capacity of the battery, but is low enough on the activation energy PDF to keep corrosion to a minimum. The iron phosphate positive terminal has a shallower discharge curve, thus retaining more capacity at 3.8V.
Battery manufacturers typically store batteries at 15°C (59°F) and a 40% state of charge (SoC), to minimize aging. Ideally, storage would take place at 4% or 5% SoC, but it must never reach 0%, or the battery may be damaged. Typically, a battery pack protection IC prevents a battery from reaching 0% SoC. But pack protection cannot prevent self-discharge and the pack protection IC itself consumes some current. Although Li-Ion batteries have less self-discharge than most other secondary batteries, the storage time is somewhat open-ended. So, 40% SoC represents a compromise between minimizing aging and preventing damage while in storage (see Figure 2).
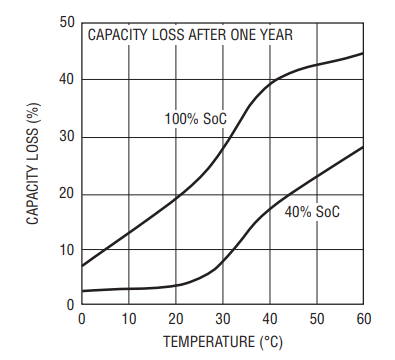
Figure 2. Yearly Capacity Loss vs Temperature and SoC for Li-Ion Batteries
In portable applications, the reduction in capacity from such a reduced SoC strategy is viewed negatively in marketing specifi cations. But it is suffi cient to detect the combination of high ambient heat and high battery SoC to implement an algorithm that minimizes aging while ensuring maximum capacity availability to the user.
Battery Conditioner Avoids Conditions that Accelerate Aging
The LTC4099 has a built-in battery conditioner that can be enabled or disabled (default) via the I2C interface. If the battery conditioner is enabled and the LTC4099 detects that the battery temperature is higher than ~60°C, it gently discharges the battery to minimize the effects of aging. The LTC4099 NTC temperature measurement is always on and available to monitor the battery temperature. This circuit is a micropower circuit, drawing only 50nA while still providing full functionality.
The amount of current used to discharge the battery follows the curve shown in Figure 3, reaching zero when the battery terminal voltage is ~3.85V. If the temperature of the battery pack drops below ~40°C and a source of energy is available, the LTC4099 once again charges the battery. Thus, the battery is protected from the worstcase battery aging conditions.
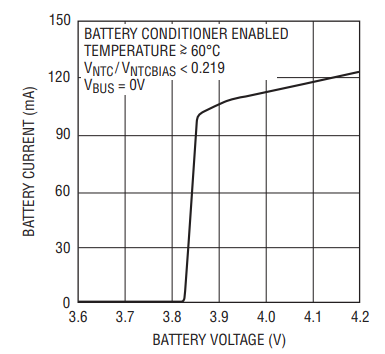
Figure 3. Battery Discharge Current vs Voltage for the LTC4099 Battery Conditioning Function
Conclusion
Although the aging of Li-Ion batteries cannot be stopped, the LTC4099’s battery conditioner ensures maximum battery life by preventing the battery-killing conditions of simultaneous high voltage and high temperature. Further, the micropower, always on NTC monitoring circuit ensures that the battery is protected from life-threatening conditions at all times.